Natriuretic Peptides and Neprilysin Inhibitors
Natriuretic Peptide Formation and Metabolism
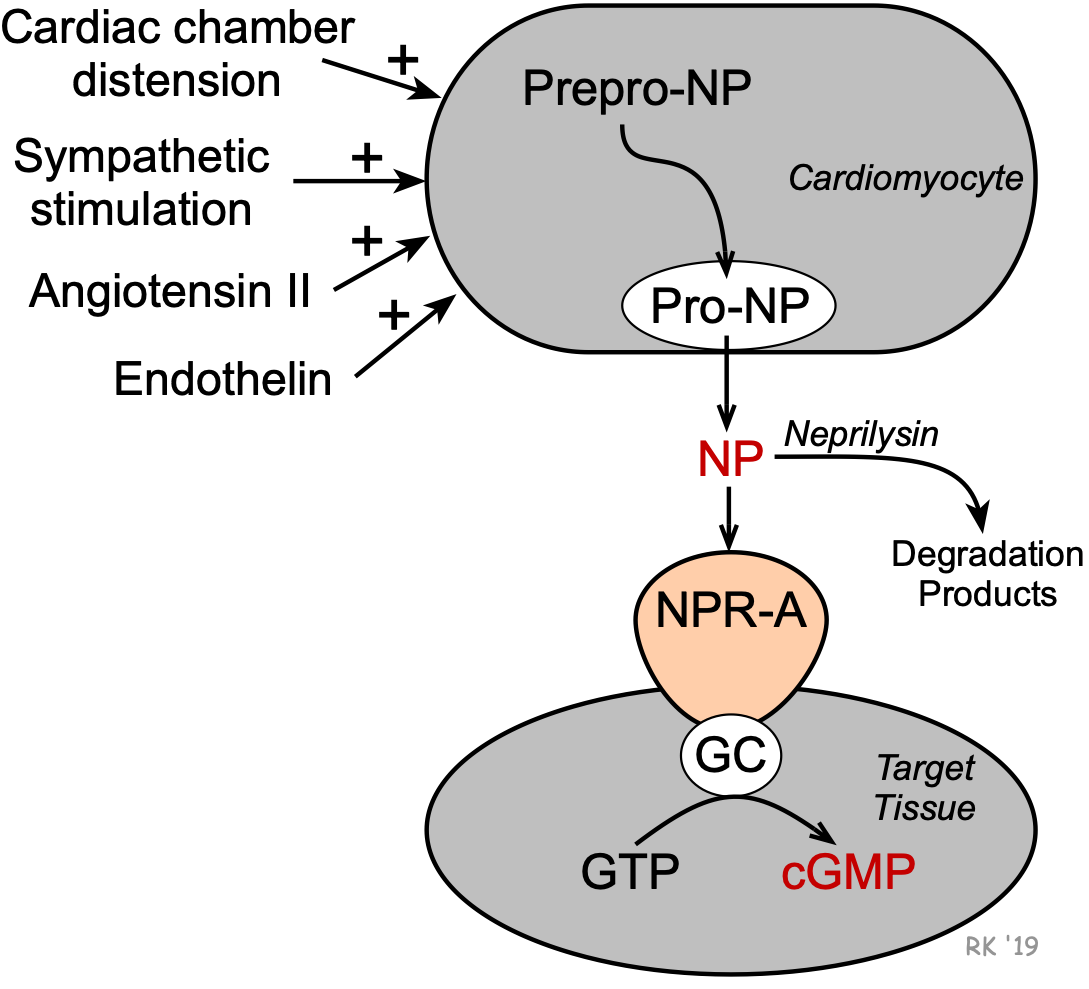 Natriuretic peptides (NPs) are peptide hormones that are synthesized by the heart, brain, and other organs. Their formation in the heart is stimulated by cardiac chamber distension, angiotensin II, endothelin, and sympathetic nerve activity (beta-adrenoceptor mediated). Formation occurs in cardiomyocytes from larger peptide precursors (prepro-NP and pro-NP). After release by the cells, these NPs circulate to their target tissues where they bind to NPR-A receptors that are linked to guanylyl cyclase and the formation of cGMP.
Natriuretic peptides (NPs) are peptide hormones that are synthesized by the heart, brain, and other organs. Their formation in the heart is stimulated by cardiac chamber distension, angiotensin II, endothelin, and sympathetic nerve activity (beta-adrenoceptor mediated). Formation occurs in cardiomyocytes from larger peptide precursors (prepro-NP and pro-NP). After release by the cells, these NPs circulate to their target tissues where they bind to NPR-A receptors that are linked to guanylyl cyclase and the formation of cGMP. Cardiovascular and Renal Effects of Natriuretic Peptides
Cardiovascular and Renal
Actions of Natriuretic Peptides
- Natriuresis
- Diuresis
- Improve glomerular filtration rate
& filtration fraction - Inhibit renin release
- ↓ circulating angiotensin II
- ↓ circulating aldosterone - Systemic vasodilation
- Arterial hypotension
- Reduced venous pressure
- Reduced pulmonary capillary
wedge pressure
Natriuretic peptides are involved in the long-term regulation of sodium and water balance, blood volume and arterial pressure. These peptide hormones decrease aldosterone release by the adrenal cortex, increase glomerular filtration rate (GFR) and filtration fraction, produce natriuresis and diuresis (potassium sparing), and decrease renin release, decreasing circulating levels of angiotensin II. These actions contribute to a reduction in blood volume and therefore central venous pressure (CVP), pulmonary capillary wedge pressure, cardiac output, and arterial blood pressure. Chronic elevations of ANP appear to decrease arterial blood pressure primarily by decreasing systemic vascular resistance. The mechanism of systemic vasodilation involves ANP receptor-mediated elevations in vascular smooth muscle cGMP and by attenuating sympathetic vascular tone. This latter mechanism may involve ANP acting upon sites within the central nervous system, as well as through inhibition of norepinephrine release by sympathetic nerve terminals. To summarize, natriuretic peptides serve as a counter-regulatory system for the renin-angiotensin-aldosterone system.
Therapeutic Use of Natriuretic Peptides in Heart Failure
Heart failure leads to activation of the renin-angiotensin-aldosterone system, which causes increased sodium and water retention by the kidneys. This renal action increases blood volume and contributes to the elevated venous pressures associated with heart failure, which can lead to pulmonary and systemic edema. Increased angiotensin II also causes systemic vasoconstriction, which increases the afterload on the left ventricle.
The rationale for using a NP in heart failure is that it should reduce pulmonary and systemic congestion and edema, and associated clinical symptoms (e.g., shortness of breath — dyspnea). NPs may also reduce the afterload on the heart by promoting systemic vasodilation, which can lead to improved ventricular ejection. A recombinant human BNP (nesiritide) was developed and evaluated to treat acute, decompensated congestive heart failure caused by systolic dysfunction. However, this drug is no longer used in the treatment of this condition because a large clinical trial failed to show improved clinical outcomes. This compound produced long-lasting hypotension among its adverse effects.
Neprilysin Inhibitors in Heart Failure
Instead of infusing natriuretic peptides, a more successful approach was found in the development of neprilysin inhibitors (e.g., sacubitril). This new drug class increases the concentration of circulating, naturally produced NPs by inhibiting their degradation. Sacubitril, when combined with an angiotensin receptor blocker (valsartan), is effective in treating acute, decompensated heart failure, and has some benefit in patients with mildly reduced ejection fractions (HFmrEF; 41 - 49%). This combined therapy enhances the inhibitory effects of naturally produced NPs on the renin-angiotensin-aldosterone system, besides blocking AT1 receptors. The combination of valsartan and sacubitril is referred to as an ARNI (angiotensin receptor neprilysin inhibitor).
Revised 11/30/2023
 Cardiovascular Physiology Concepts, 3rd edition textbook, Published by Wolters Kluwer (2021)
Cardiovascular Physiology Concepts, 3rd edition textbook, Published by Wolters Kluwer (2021) Normal and Abnormal Blood Pressure, published by Richard E. Klabunde (2013)
Normal and Abnormal Blood Pressure, published by Richard E. Klabunde (2013)