Renin Inhibitors
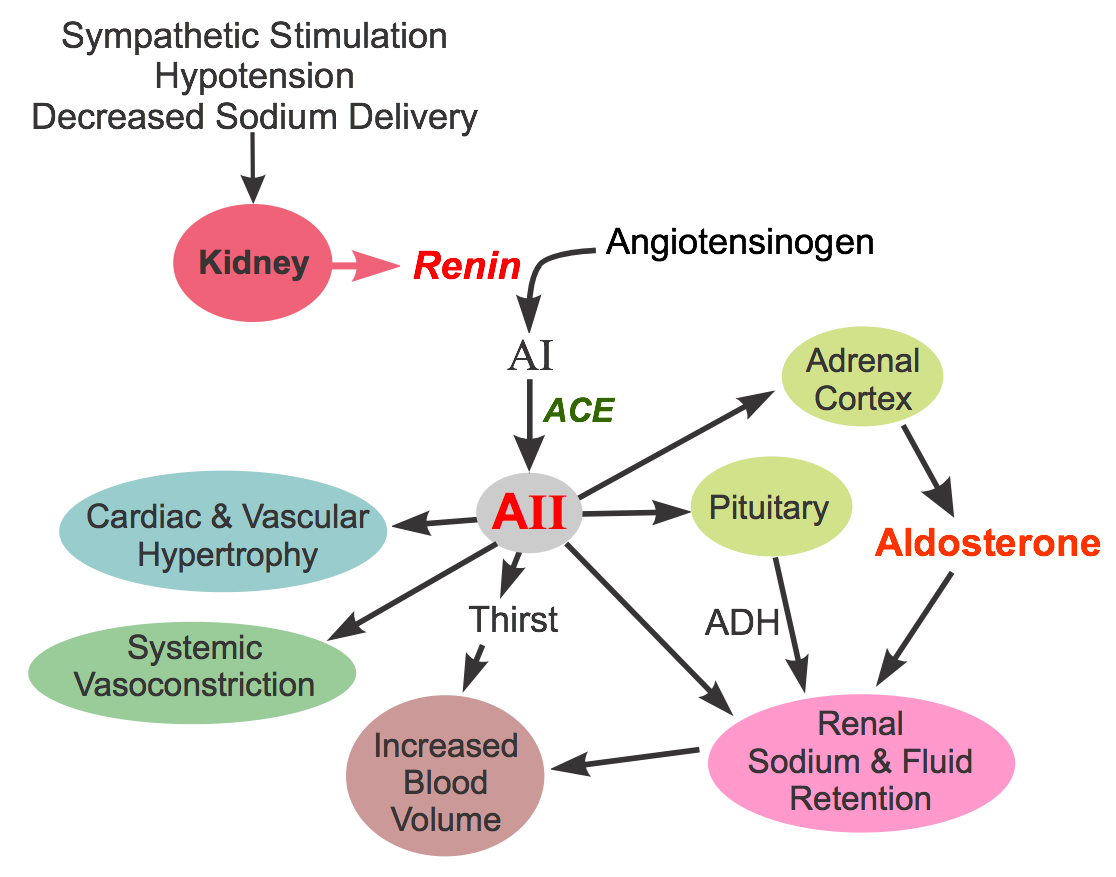 Renin inhibitors are one of four classes of compounds that affect the renin-angiotensin-aldosterone system, the other three being angiotensin converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs) and aldosterone receptor antagonists. Renin inhibitors produce vasodilation by inhibiting the activity of renin, which stimulates angiotensin II formation. This substance is a proteolytic enzyme that is released by the kidneys in response to sympathetic activation, hypotension, and decreased sodium delivery to the distal renal tubule (click here for more details). Once released into the circulation, renin acts on circulating angiotensinogen to form angiotensin I. Angiotensin I is then converted to angiotensin II by angiotensin converting enzyme (ACE). Angiotensin II has several important actions, including vasoconstriction, stimulation of aldosterone, renal retention of sodium and water, enhancing sympathetic activity by increasing norepinephrine release by sympathetic nerves, stimulating antidiuretic hormone (vasopressin) release and thirst, and promoting cardiac and vascular hypertrophy.
Renin inhibitors are one of four classes of compounds that affect the renin-angiotensin-aldosterone system, the other three being angiotensin converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs) and aldosterone receptor antagonists. Renin inhibitors produce vasodilation by inhibiting the activity of renin, which stimulates angiotensin II formation. This substance is a proteolytic enzyme that is released by the kidneys in response to sympathetic activation, hypotension, and decreased sodium delivery to the distal renal tubule (click here for more details). Once released into the circulation, renin acts on circulating angiotensinogen to form angiotensin I. Angiotensin I is then converted to angiotensin II by angiotensin converting enzyme (ACE). Angiotensin II has several important actions, including vasoconstriction, stimulation of aldosterone, renal retention of sodium and water, enhancing sympathetic activity by increasing norepinephrine release by sympathetic nerves, stimulating antidiuretic hormone (vasopressin) release and thirst, and promoting cardiac and vascular hypertrophy.
Renin inhibitors have the following actions, which are like those produced by ACEIs and ARBs: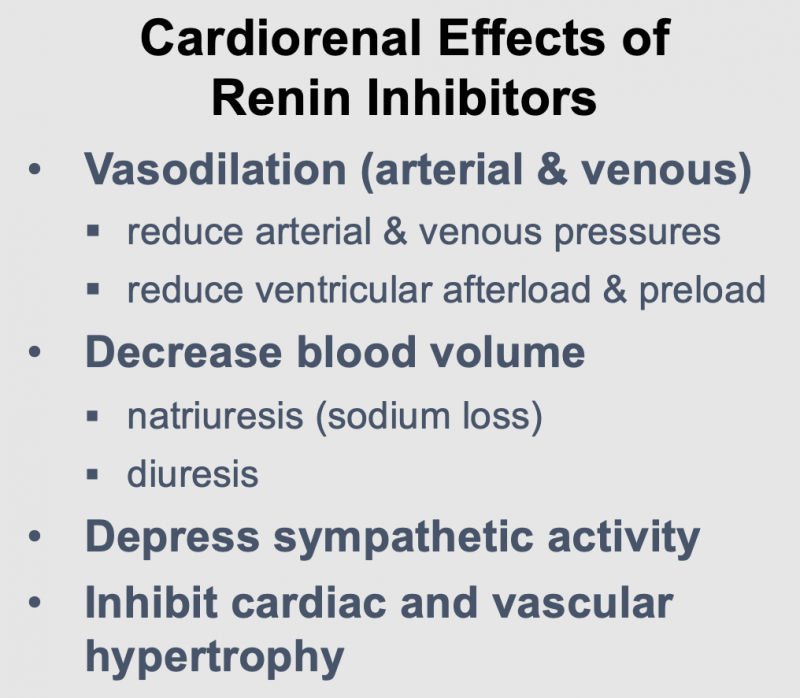
- Dilate arteries and veins by blocking angiotensin II formation. This vasodilation reduces arterial pressure, preload and afterload on the heart.
- Downregulate sympathetic adrenergic activity by blocking the facilitating effects of angiotensin II on sympathetic nerve release and reuptake of norepinephrine.
- Promote renal excretion of sodium and water (natriuretic and diuretic effects) by blocking the effects of angiotensin II in the kidney and by blocking angiotensin II stimulation of aldosterone secretion. This reduces blood volume, venous pressure, and arterial pressure.
- Inhibit cardiac and vascular remodeling associated with chronic hypertension, heart failure, and myocardial infarction.
Specific Drugs and Therapeutic Uses
Aliskiren is a renin inhibitor that was approved to treat hypertension by the U.S. FDA in 2007. Aliskiren is orally active, has a half-life of about 24 hours, and is dosed once per day. Because of its relatively long half-life, it takes about 1 week of dosing to achieve a near maximal antihypertensive effect. It is metabolized by the liver and excreted by the kidneys. Normal therapeutic concentrations of aliskiren reduce plasma renin activity by 50-80%. It is effective in monotherapy. When used with thiazide diuretics or ARBs, the antihypertensive effects are additive.
Side Effects and Contraindications
Aliskiren alone, like ACEIs and ARBs, has a relatively low incidence of side effects and is well-tolerated. Aliskiren has dose-related gastrointestinal adverse effects in some patients; diarrhea is observed in less than 3% of patients. The incidence of cough is much lower in patients taking aliskiren than those taking ACEIs. Angioedema (life-threatening airway swelling and obstruction) can occur in patients taking aliskiren (as can occur with ACEI and ARB treatment), although fewer than 1% of patients develop this condition. When administered with an ACEI, aliskiren can produce hyperkalemia, especially in diabetic patients. The ALTITUDE trial noted increased adverse events (non-fatal stroke, renal complications, hyperkalemia, hypotension) with no apparent additional benefits when added to treatment with an ACEI or ARB in diabetic patients.
As with ACEIs, aliskiren should not be administered anytime during pregnancy, particularly in second and third trimesters because of fetal and neonatal injury, and risk of birth defects.
Revised 01/28/2024
 Cardiovascular Physiology Concepts, 3rd edition textbook, Published by Wolters Kluwer (2021)
Cardiovascular Physiology Concepts, 3rd edition textbook, Published by Wolters Kluwer (2021) Normal and Abnormal Blood Pressure, published by Richard E. Klabunde (2013)
Normal and Abnormal Blood Pressure, published by Richard E. Klabunde (2013)